Hydrogen tablets produce hydrogen gas through the reaction of elemental magnesium (Mg) and water:
Mg + 2H2O => Mg(OH)2 + H2
The production of the gas bubbles can be easily observed when placing a tablet in a clear container, and in a closed container, the pressure will rise as the volume of H2 gas produced increases.
Distinguishing Between "Produced" and "Dissolved" Hydrogen Gas:
Because each tablet contains a fixed amount of magnesium metal, usually in the range of 50 to 80 milligrams, the total amount of H2 gas which can be produced from each tablet is also fixed. Although the theoretical maximum amount of H2 gas which can be produced from one tablet (under ideal conditions) can be calculated based upon the amount of magnesium in the tablet (usually indicated on the label), not all of the H2 gas produced will actually dissolve into the water. This is because a portion of the H2 gas will escape into the air and be wasted. The percentage of H2 produced which actually dissolves into the water is typically in the 25% to 50% range. This percentage can be influenced by factors such as temperature, time & pressure. Only the dissolved H2 gas can be considered when calculating ingestible H2 levels.
Converting the Amount of Ingestible H2 based on PPM Reading and Container Size:
In order to calculate the amount of ingestible dissolved H2 present in a given container using the H2Blue PPM reading, the size of the container into which the tablet was placed (and from which the 6ml test sample is taken) must be taken into consideration. This is because it takes fewer milligrams of H2 to produce 1PPM in smaller amounts of water than it does in 1 liter of water. The following graphic illustrates this concept:
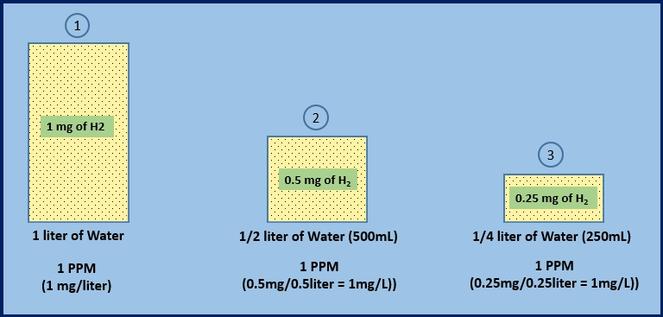
In this example, a 6ml sample taken from each container will measure the same, 1PPM (10 drops of H2Blue). But, these containers DO NOT contain the same AMOUNT of dissolved H2. As the volume of water is halved, so too is the amount of dissolved H2, resulting in the SAME concentration in each container. From this example, it can be seen that PPM's DO NOT tell the entire story about how much H2 will be ingested when drinking the H2 water being sampled. We must relate the test sample to the size of the container from which it was taken to know how much H2 will be ingested when the water is consumed.
Calculating the Theoretical Maximum Amounts of H2 which can be
Produced and Dissolved:
Utilizing the number of milligrams of Magnesium contained in the tablet,
we can:
- Calculate the theoretical maximum amount of H2 which can be produced
From which we can then also:
- Approximate the maximum level of dissolved H2 (PPM) and ingestible H2
(mg) which can be expected.
When calculating ingestible H2 levels, it is important to remember that
only DISSOLVED H2 can be considered (regardless of how much H2 is
produced). A few variables come into play which have an impact on the
percentage of H2 gas which ultimately dissolves into the water. For any
given tablet size, these variables include:
-
Whether or not the container can maintain pressure - pressure helps H2
dissolve better.
- Materials from which the container is constructed - less-porous
materials hold H2 better.
- The amount of time the tablet is permitted to dissolve - more time
(hours) is generally better than less time (minutes)
- The temperature of the water - H2 will dissolve better in cold water
While a larger container will produce lower PPM readings, the amount of
ingestible H
2 will not necessarily be less.
Table 1 below shows the maximum H
2 production possible, and the levels
of measured PPM and ingestible H
2 one can expect based on
some tablet sizes, container volumes, and dissolved H
2 percentages:
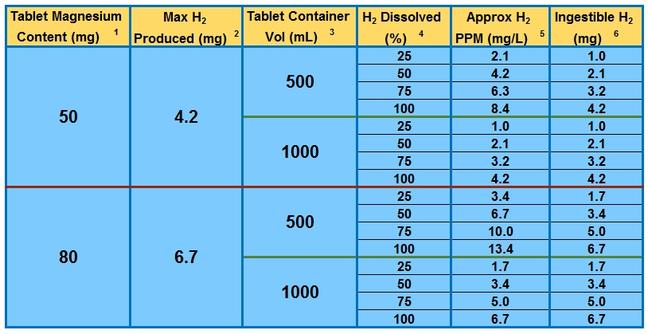
Table 1
How to use this table:
- Select the value in column 1 corresponding to the amount of magnesium in your tablet, usually found on the label (if using two tablets, double the results shown in columns 2,5 & 6)
- Column 2 shows the maximum amount of hydrogen gas which can be produced from the tablet (under ideal conditions), based on the reaction stoichiometry and molecular weights.
- Select the value in column 3 corresponding to the volume of water into which the tablet is to be dissolved.
- Select the value in column 4 corresponding to the anticipated percentage of dissolved hydrogen. Values will vary depending on preparation method, but will typically be in the 20% to 50% range.
- Column 5 will give the approximate PPM which you would expect to measure based upon the amount of magnesium in the tablet, container volume, and % of the produced H2 which dissolves.
- Column 6 will give the approximate amount of H2 which will be ingested if one drinks the contents of the container.
Example: One 50mg tablet is capable of producing 4.2mg of H
2. If it is placed into a 500mL container, allowed to dissolve for 20 minutes, and we assume that 25% of the H
2 which can be produced will dissolve, then a concentration of approximately 2.1PPM will be measured, and approximately 1mg of H
2 will be ingested when consuming the entire contents.